FDA clears Pfizer boosters for high-risk groups
President Biden sets a new 70% vaccination goal: this time, it's the world
Quite a bit to catch up on today. This afternoon, The U.S. Food and Drug Administration endorsed the recommendation made by its outside advisors last week to permit booster shots of the Pfizer COVID-19 vaccine for those at highest risk.
Specifically, the FDA’s Emergency Use Authorization now expands to allow a booster shot at least six months after the initial two-dose regimen for these groups:
65 years and older;
18-64-year-olds at high risk of severe COVID-19; and
18-64-year-olds whose frequent institutional or occupational exposure to SARS-CoV-2 puts them at high risk of serious complications of COVID-19 including severe COVID-19.
Local vaccination sites likely will start giving booster doses as soon as they get guidance from the CDC, which is expected within the next day or two.
In other news:
Kicking off meetings with world leaders, President Biden called on other nations to join the USA so that 70% of the world can get vaccinated against COVID-19 within the next 12 months.
The White House also announced new commitments to improve the global supply chain for pharmaceuticals, PPE and other pandemic essentials.
The USA will purchase and distribute an additional 500 million doses of Pfizer COVID-19 vaccine starting in January, bringing the total U.S. vaccine donation to 1.1 billion doses overall by the end of next year.
Johnson & Johnson says its test of giving a second shot six months after the first one increases the body’s immune response 12 times. A second shot within two months increases the response but only four to six times.
The company also reported that its “real world” data shows no waning of immune response or reduced efficacy.
Based on actual experience in the USA since the EUA was granted, the one-shot J&J vaccine has an efficacy rate of 74% against COVID-19 infections, 89% against COVID-19 hospitalizations and 83% against COVID-19 deaths. The company says this includes observations after the delta variant became prevalent.
The data was published in a news release and has not yet been peer-reviewed.
A trial has begun in Austria over a COVID-19 outbreak at a ski resort, which turned out to be one of the first “super spreader” events of the pandemic.
If you’re wondering why the anti-vaccine crowd is going crazy trying to get doses of the anti-parasite medicine ivermectin, this article traces some of the frenzy to a couple of doctors who championed the unsubstantiated claims — and have ties to an organization that makes money selling merchandise promoting the drug.
With hospitalizations and new cases still at high levels in much of the nation, some regions report shortages of life-saving monoclonal antibody treatments.
Most people would not need the mABs if they would have gotten vaccinated. Vaccines prevent COVID-19 infection. Monoclonal antibodies treat people who are at high-risk of getting severely sick from COVID-19 infection.
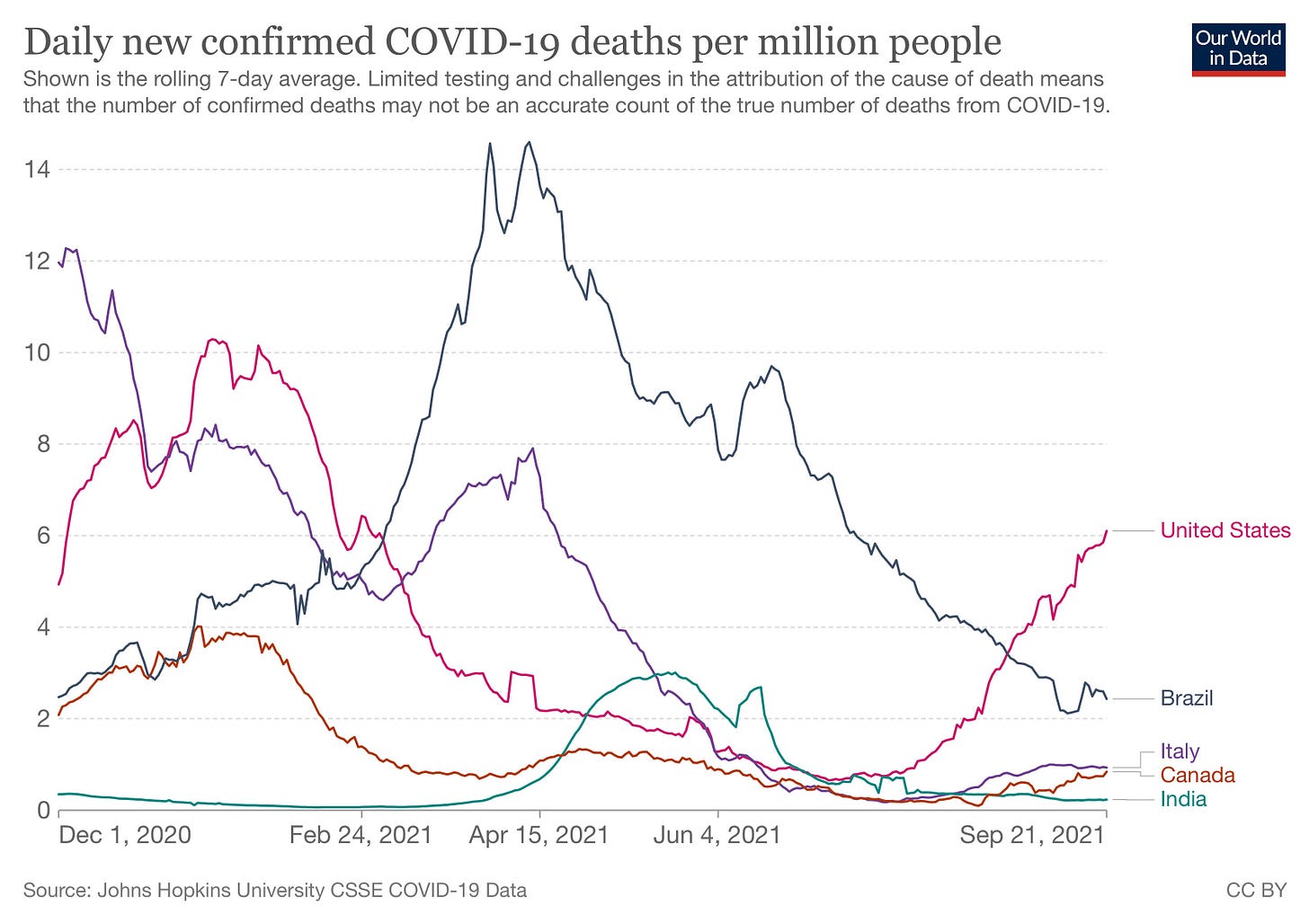
Factcheck: How’s the USA doing? Here’s a chart showing deaths since December, when vaccines started to roll out.
And for the travelers among us: there are lots of questions about cancellation rules and travel insurance. This post on ThePointsGuy.com is a good overview on how travel insurance works and how to decide whether it’s worth buying because of COVID-19.
There is a lot of data available on various aspects of the pandemic. What are some of your questions? Let’s see if we can find good answers.