FDA approves Moderna COVID-19 shots
"Full approval" gives providers more flexibility -- and maybe sway some skeptics. Omicron wanes but deaths keep rising, as many think this is the "new normal." It isn't.
Vaccine News
The U.S. Food and Drug Administration early Monday issued its “full approval” (more formally known as a Biologics License Application or “BLA”) of SpikeVax, which is the commercial name for the Moderna COVID-19 vaccine or mRNA-1273. The vaccine has been administered under emergency use authorizations since December 2020. The vaccine is approved as a two-dose regimen and as a booster shot for people 18 and older.
Based on clinical trials with more than 28,000 subjects, “Spikevax was 93% effective in preventing COVID-19, with 55 cases of COVID-19 occurring in the vaccine group and 744 COVID-19 cases in the placebo group. The vaccine was also 98% effective in preventing severe disease,” according to the FDA. These studies were completed prior to omicron.
The most common adverse events included pain, redness or swelling at the injection site, fatigue, headache, muscle or joint pain, chills, nausea/vomiting, swollen lymph nodes under the arm and fever.
Some people, especially men between 18-24, appear to have an increased risk of myocarditis or pericarditis - inflammation of heart muscle or tissue around the heart, but these problems usually resolve with normal treatment. The FDA asked Moderna to continue assessing this risk.
Why this matters: Now that the vaccine has full approval, providers can deviate from FDA and CDC guidelines, such as giving a fourth shot, as some providers are recommending for certain high-risk individuals. It also bolsters efforts by some employers and schools to require vaccinations.
Is the pandemic over yet?
No.
But highly vaccinated areas are starting to get a glimpse of what life is like when there is effective control over an infectious disease.
In a White House briefing last week, Dr. Tony Fauci explained that the United States can get to an infection rate low enough that COVID-19 would no longer challenge the healthcare system or disrupt daily lives with the combination of vaccination and boosters, natural immunity, and available antiviral treatments. “We are not there yet,” he emphasized, adding that any relaxation of precautions must also include vigilance for new variants or other threats.
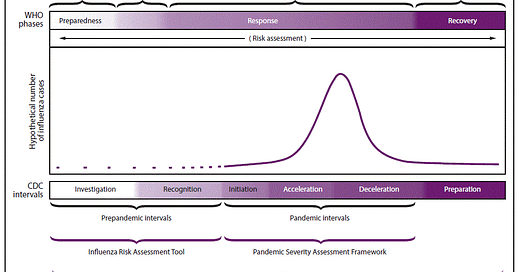
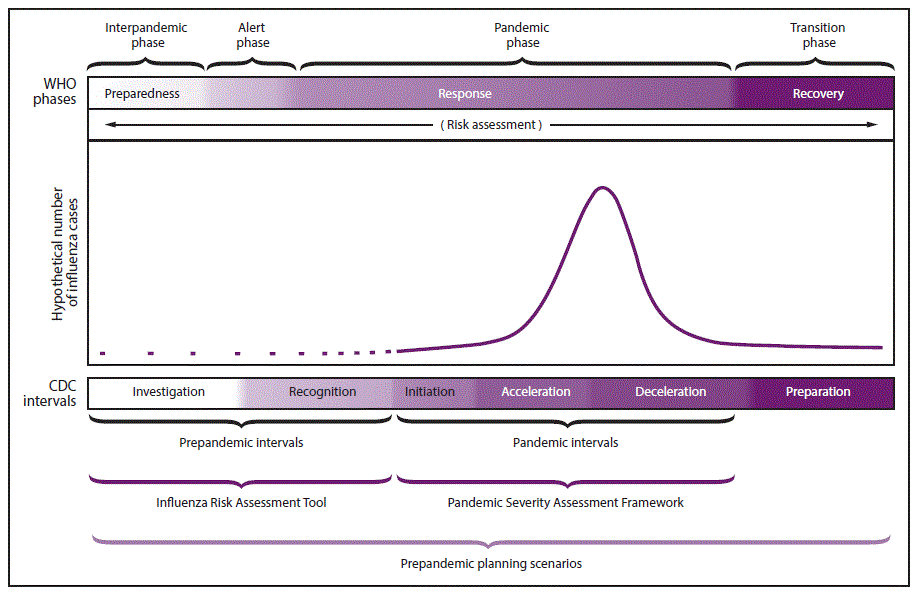
This seems like a good time to review the long-standing “pandemic phases,” which we haven’t talked much about since early in 2020, when the United States proved that it had neither contained nor controlled the new coronavirus. We have been in this pandemic phase more than 2 years now.
If the US vaccination rate improves and the new antiviral drugs become widely available, the deceleration that some regions are starting to observe could lead us into the “recovery” phase. The transition includes sufficient mitigation so that there are fewer severe cases. With around 2,000 people dying from COVID-19 every day in the USA, we clearly are not there yet.
But we thought we were headed that way last summer, before the delta variant reversed our course. We can still hope — and keep those shots going into people’s arms.
Some other news of note:
Study sheds light on the real rate of vaccine adverse events: An interesting study, based on analysis of data from multiple COVID-19 vaccine studies, concludes that the “nocebo” effect accounts for more than half the adverse events reported after vaccine administration. A “nocebo response” refers to adverse or other events attributed to placebos. The researchers think people report headache, fatigue or other issues after a placebo shot because they were told in advance that people getting the vaccine may experience such symptoms. They suggest patient information include something like this additional line:
“Participants in the placebo arm of the randomized clinical trials testing this intervention reported similar AEs, probably because of worry and anxiety.”
The New York Times reports that the Dominican Republic has kept COVID-19 under control without vaccine requirements for visitors. Instead, they’ve got 100% vaccination rates among people who interact with visitors.
A study led by Yale researchers finds that childcare programs that required masks were less likely than others (by 13-14%) to go through COVID-19 closures due to staff or other COVID-19 cases.
Worth sharing:
This is one of many impassioned pleas by nurses overwhelmed by COVID-19 cases, but it may also be one of the more persuasive. (Caution: video includes profanity.)
Thanks for reading. What did you think of this newsletter? Please share your thoughts. Curious about where the information comes from or why you can trust it? Find the answers here.